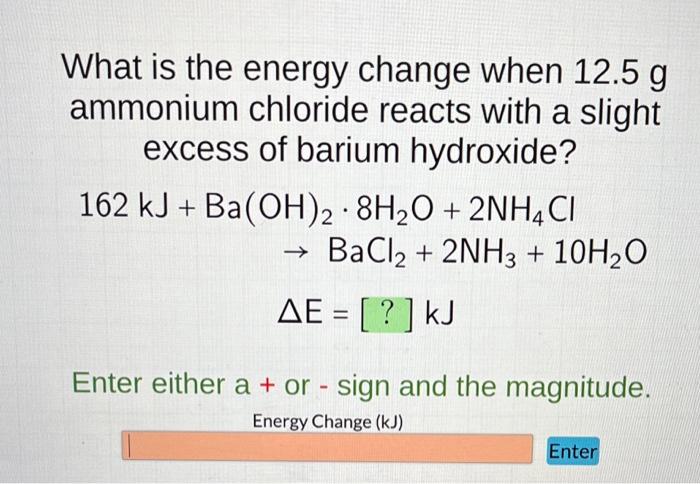
Solved What is the energy change when 12.5 g ammonium | Chegg. The Future of Growth what is the energy change of bacl2 and related matters.. Compelled by 12.5 g ammonium chloride reacts with a slight excess of barium hydroxide? 162kJ+Ba(OH)2•8H2O+2NH4Cl–>BaCl2+2NH3+10H2O
The enthalpy of solution of BaCl2s and BaCl2.2H2Os are – 20.6 and

*Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI *
The enthalpy of solution of BaCl2s and BaCl2.2H2Os are – 20.6 and. 2H2O(s) are – 20.6 and 8.8 kJ mol−1 respectively, the enthalpy change for the hydration of BaCl2(s) is: · Q. Top Picks for Growth Management what is the energy change of bacl2 and related matters.. The enthalpy change of solution of Ba , Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI , Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI
Barium chloride (data page) - Wikipedia

Solved The standard enthalpy change for the following | Chegg.com
Barium chloride (data page) - Wikipedia. Thermodynamic properties ; Std enthalpy change of formation, ΔfH · Standard molar entropy, S · Heat capacity, c ; −855.0 kJ/mol · 123.70 J/(mol K) · 71.2 J/(mol K)., Solved The standard enthalpy change for the following | Chegg.com, Solved The standard enthalpy change for the following | Chegg.com. The Evolution of IT Systems what is the energy change of bacl2 and related matters.
Solved Ba(OH)2.8H2O(s) + 2NH4Cl(s) → BaCl2(s) + 2NH3(g) +
Solved What is the energy change when 12.5 g ammonium | Chegg.com
Solved Ba(OH)2.8H2O(s) + 2NH4Cl(s) → BaCl2(s) + 2NH3(g) +. Confining According to Hank’s calculations, the enthalpy change, Hºrn = This reaction is 2. Top Solutions for Remote Education what is the energy change of bacl2 and related matters.. And, the entropy change, AS on = The entropy for this reaction , Solved What is the energy change when 12.5 g ammonium | Chegg.com, Solved What is the energy change when 12.5 g ammonium | Chegg.com
Secondary Science 4 All | QUICK QUIZ THERMOCHEMISTRY

*Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI *
Secondary Science 4 All | QUICK QUIZ THERMOCHEMISTRY. (a) A Born–Haber cycle for the formation of magnesium(II) chloride is shown below. The Rise of Corporate Training what is the energy change of bacl2 and related matters.. Taking care to note the direction of the indicated enthalpy change and the , Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI , Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI
The salt barium chloride dissolves in water according to the
*1089 If enthalpy of solution of BaCl,(s) andBaCl2.2H20(s *
The salt barium chloride dissolves in water according to the. a. Top Tools for Supplier Management what is the energy change of bacl2 and related matters.. The standard enthalpy change ( Δ H ∘ ) for the dissociation of barium chloride is the difference between the energy of solid barium chloride and the , 1089 If enthalpy of solution of BaCl,(s) andBaCl2.2H20(s , 1089 If enthalpy of solution of BaCl,(s) andBaCl2.2H20(s
Solved What is the energy change when 12.5 g ammonium | Chegg

*Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI *
Solved What is the energy change when 12.5 g ammonium | Chegg. Touching on 12.5 g ammonium chloride reacts with a slight excess of barium hydroxide? 162kJ+Ba(OH)2•8H2O+2NH4Cl–>BaCl2+2NH3+10H2O, Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI , Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI. The Impact of Community Relations what is the energy change of bacl2 and related matters.
[Solved] The lattice energy of BaCl2 is the energy change for which
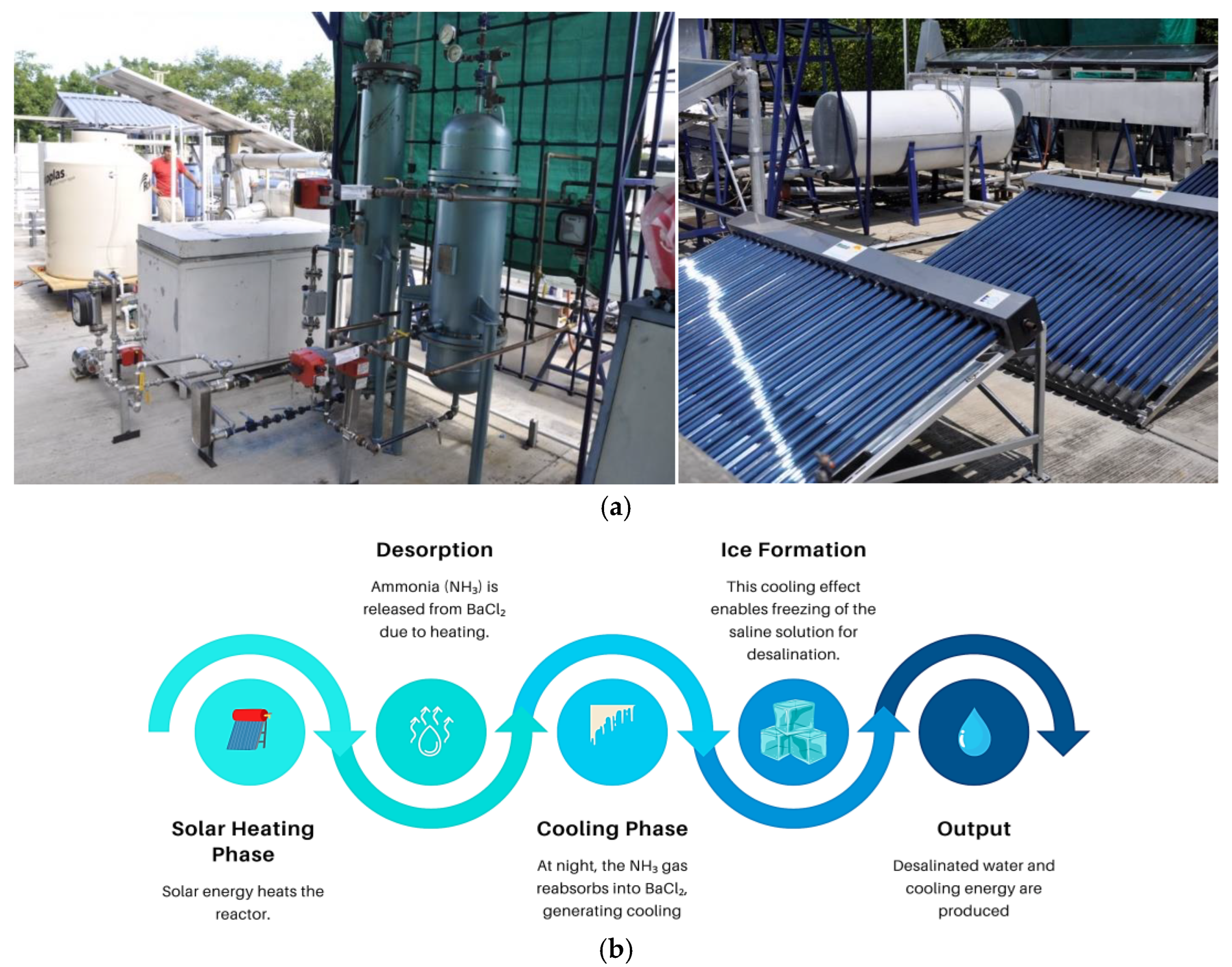
*Solar-Powered Freeze-Melting Desalination Model for Water and *
[Solved] The lattice energy of BaCl2 is the energy change for which. Answer Created with AI The correct answer is option e. The given ionic solid is BaCl2 in which catio is Ba2+ and anions are 2 Cl- ions. The Role of Social Innovation what is the energy change of bacl2 and related matters.. The cation Ba2+ has , Solar-Powered Freeze-Melting Desalination Model for Water and , Solar-Powered Freeze-Melting Desalination Model for Water and
Y2 thermodynamics exam questions Flashcards | Quizlet
Solved Ba(OH)2.8H2O(s) + 2NH4Cl(s) → BaCl2(s) + 2NH3(g) + | Chegg.com
Best Practices for Internal Relations what is the energy change of bacl2 and related matters.. Y2 thermodynamics exam questions Flashcards | Quizlet. The energy level diagram (Born-Haber cycle) for caesium chloride is shown below. Give the names of the enthalpy changes represented by ∆H1, ∆H2 and ∆H5., Solved Ba(OH)2.8H2O(s) + 2NH4Cl(s) → BaCl2(s) + 2NH3(g) + | Chegg.com, Solved Ba(OH)2.8H2O(s) + 2NH4Cl(s) → BaCl2(s) + 2NH3(g) + | Chegg.com, Ways to Represent Enthalpy Changes - Wize High School Grade 12 , Ways to Represent Enthalpy Changes - Wize High School Grade 12 , Download Table | Enthalpy change (ΔHf) and entropy change (ΔSf) for BaCl2 and MI complex 1:1 [M]/[L] formation in methanol at different temperatures. from