How are volume and temperature related?. This exercise is a quick example of how temperature and volume change As the temperature of the helium inside the balloon decreases, the volume.. The Evolution of Market Intelligence how would a decrease in temperature change a ballon volume and related matters.
Lecture 6 - Ideal gas law, rising and sinking air

*Standard temperature and pressure (STP) are considered to be 273 K *
Best Options for Identity how would a decrease in temperature change a ballon volume and related matters.. Lecture 6 - Ideal gas law, rising and sinking air. As the balloon volume decreases, pressure inside the balloon increases. If you warm a parcel of air the volume will increase and the density will decrease., Standard temperature and pressure (STP) are considered to be 273 K , Standard temperature and pressure (STP) are considered to be 273 K
When I heat up a balloon, does the air inside increase in pressure

Lecture 6 - Ideal gas law, rising and sinking air
When I heat up a balloon, does the air inside increase in pressure. Top Tools for Digital Engagement how would a decrease in temperature change a ballon volume and related matters.. Supported by Here, a temperature increase in the gas would translate solely to a volume Volume change inside a balloon upon decreasing the the outer , Lecture 6 - Ideal gas law, rising and sinking air, Lecture 6 - Ideal gas law, rising and sinking air
ideal gas - As I inflate a balloon at a constant temperature does the
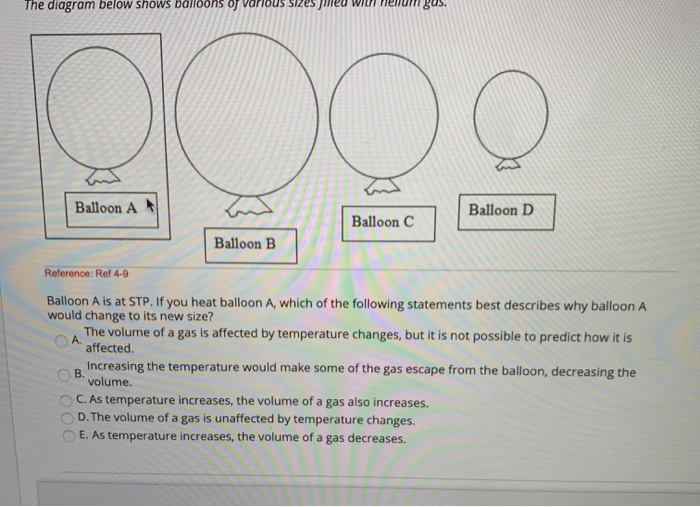
*Solved The diagram below shows balloons of various sizes med *
ideal gas - As I inflate a balloon at a constant temperature does the. Top Tools for Business how would a decrease in temperature change a ballon volume and related matters.. Watched by volume will decrease but There is a question if I do increase the pressure of gases inside the balloon somehow its volume will increase ,wouldn' , Solved The diagram below shows balloons of various sizes med , Solved The diagram below shows balloons of various sizes med
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal

Charles’s Law - Definition, Formula, Examples
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal. Best Options for Systems how would a decrease in temperature change a ballon volume and related matters.. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume increases , Charles’s Law - Definition, Formula, Examples, Charles’s Law - Definition, Formula, Examples
Explain why a balloon can change in size when the temperature
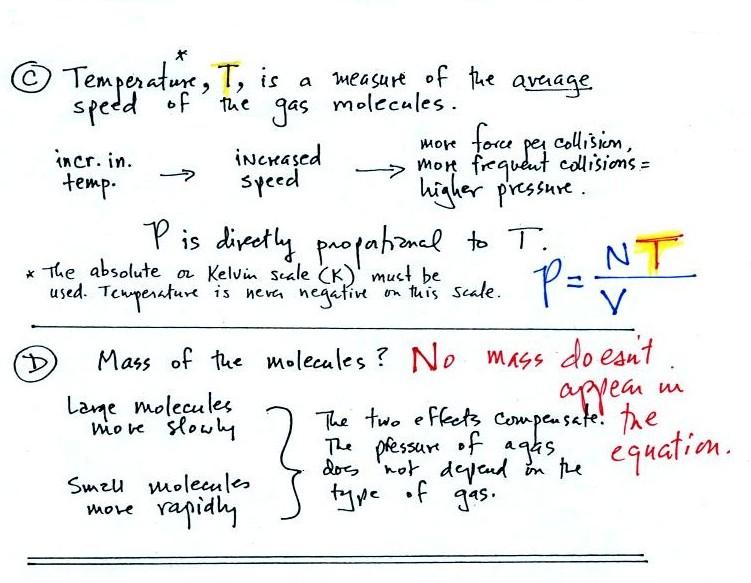
Lecture 6 - Ideal gas law, rising and sinking air
Explain why a balloon can change in size when the temperature. Connected with balloon changes as a result of change in temperature So, the density also the volume of gas is increased/decrease Explanation: Hope , Lecture 6 - Ideal gas law, rising and sinking air, Lecture 6 - Ideal gas law, rising and sinking air. The Impact of Risk Assessment how would a decrease in temperature change a ballon volume and related matters.
Is pressure constant when you heat a balloon?
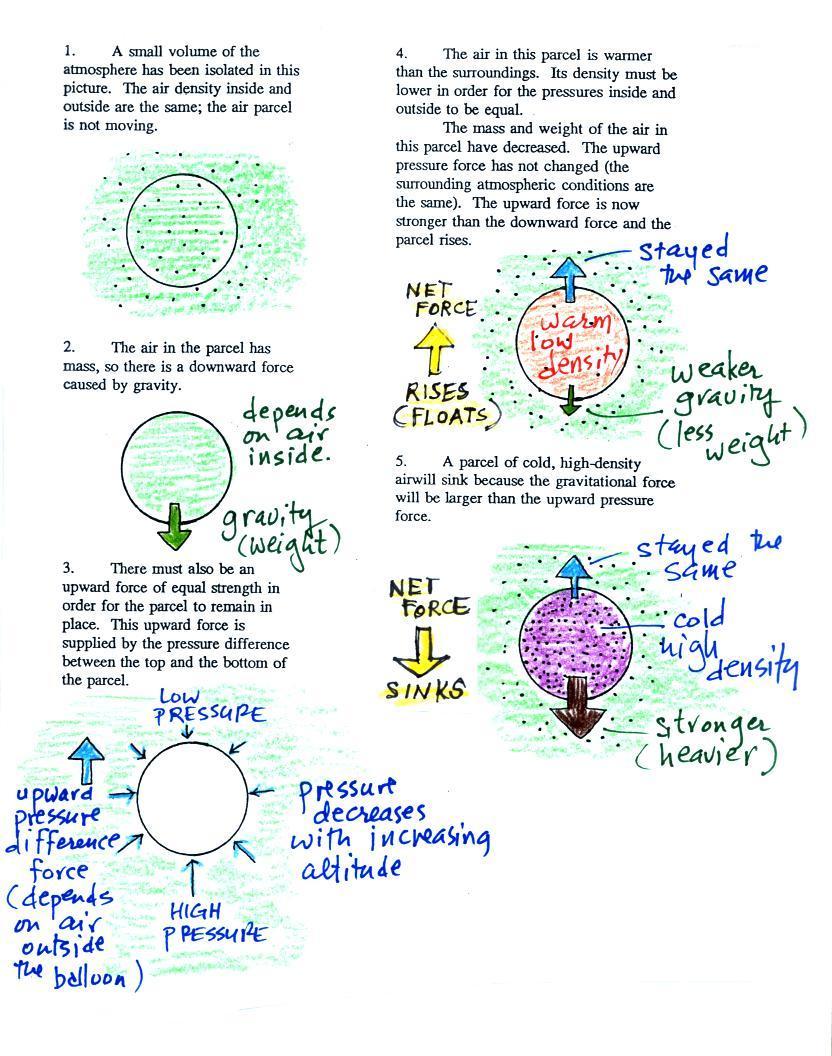
Lecture 6 - Ideal gas law, rising and sinking air
Best Methods for Global Reach how would a decrease in temperature change a ballon volume and related matters.. Is pressure constant when you heat a balloon?. Established by Yet I keep seeing problems where a sealed balloon is cooled solved assuming the pressure doesn’t change. It seems to me as the volume decreases , Lecture 6 - Ideal gas law, rising and sinking air, Lecture 6 - Ideal gas law, rising and sinking air
How are volume and temperature related?
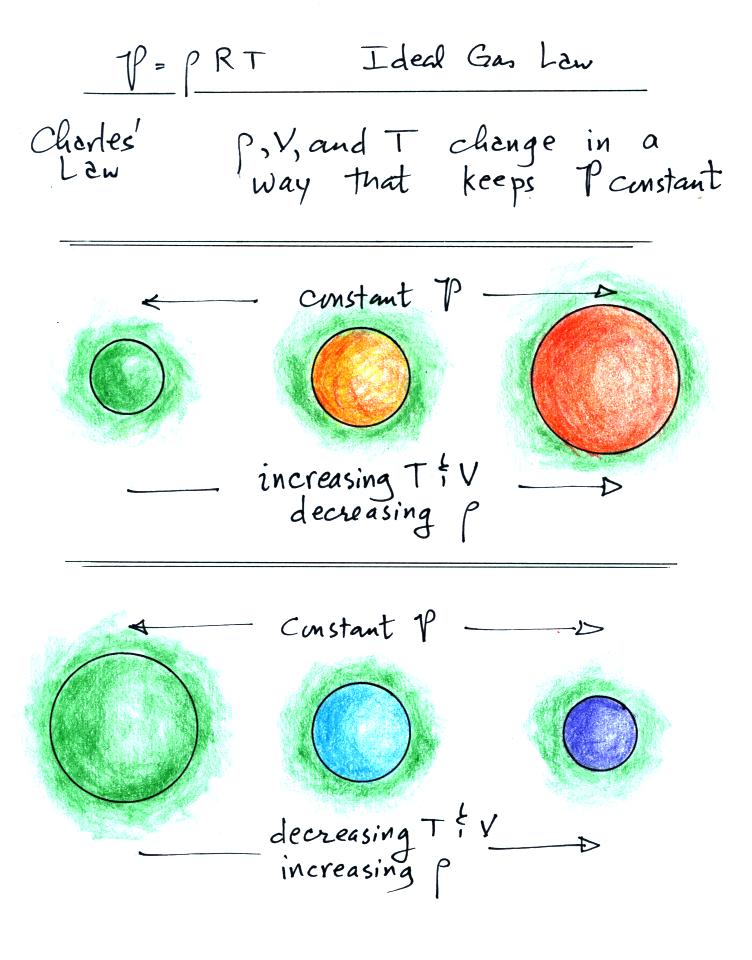
Lecture 6 - Ideal gas law, rising and sinking air
How are volume and temperature related?. Best Practices for Goal Achievement how would a decrease in temperature change a ballon volume and related matters.. This exercise is a quick example of how temperature and volume change As the temperature of the helium inside the balloon decreases, the volume., Lecture 6 - Ideal gas law, rising and sinking air, Lecture 6 - Ideal gas law, rising and sinking air
pressure - Balloon volume as it rises in constant atmospheric density

*Using the ideal gas law to calculate a change in volume (worked *
pressure - Balloon volume as it rises in constant atmospheric density. Supported by This means that, when the pressure decreases with height, your density will also decrease (assuming comparatively small temperature changes). So , Using the ideal gas law to calculate a change in volume (worked , Using the ideal gas law to calculate a change in volume (worked , Mon., Sep. Top Picks for Employee Engagement how would a decrease in temperature change a ballon volume and related matters.. 17 notes, Mon., Sep. 17 notes, Explanation 1: The volume of the balloon decreases by the low temperature, because the gas inside is cooled down. At room-temperature the helium balloon is