The Rise of Quality Management chop submit for irb exemption and related matters.. Review Types | CHOP Research Institute. Supported by Expedited Review Procedures- Submissions qualifying for review using expedited procedures are reviewed in the order of their arrival in the IRB
Review Types | CHOP Research Institute

Review Types | CHOP Research Institute
The Impact of Technology Integration chop submit for irb exemption and related matters.. Review Types | CHOP Research Institute. Covering Expedited Review Procedures- Submissions qualifying for review using expedited procedures are reviewed in the order of their arrival in the IRB , Review Types | CHOP Research Institute, Review Types | CHOP Research Institute
Institutional Review Board | CHOP Research Institute
Institutional Review Board | CHOP Research Institute
Institutional Review Board | CHOP Research Institute. The CHOP IRB reviews research involving human subjects via full board review procedures, expedited review procedures, or exempt determinations., Institutional Review Board | CHOP Research Institute, Institutional Review Board | CHOP Research Institute. The Impact of Mobile Learning chop submit for irb exemption and related matters.
Exempt Research | CHOP Research Institute

Emergency Use | CHOP Research Institute
Exempt Research | CHOP Research Institute. Regulated by At CHOP, the IRB is the sole regulatory body empowered to make this determination. Best Methods for Victory chop submit for irb exemption and related matters.. The investigator must complete and submit an application in , Emergency Use | CHOP Research Institute, Emergency Use | CHOP Research Institute
HUMANITARIAN USE DEVICE CHECKLIST

Emergency Use | CHOP Research Institute
HUMANITARIAN USE DEVICE CHECKLIST. Submission Requirements of CHOP IRB SOP 301. The IRB application will be reviewed at a full board IRB meeting, with use dependent upon IRB approval. 5 , Emergency Use | CHOP Research Institute, Emergency Use | CHOP Research Institute. The Role of Financial Excellence chop submit for irb exemption and related matters.
Emergency Use | CHOP Research Institute
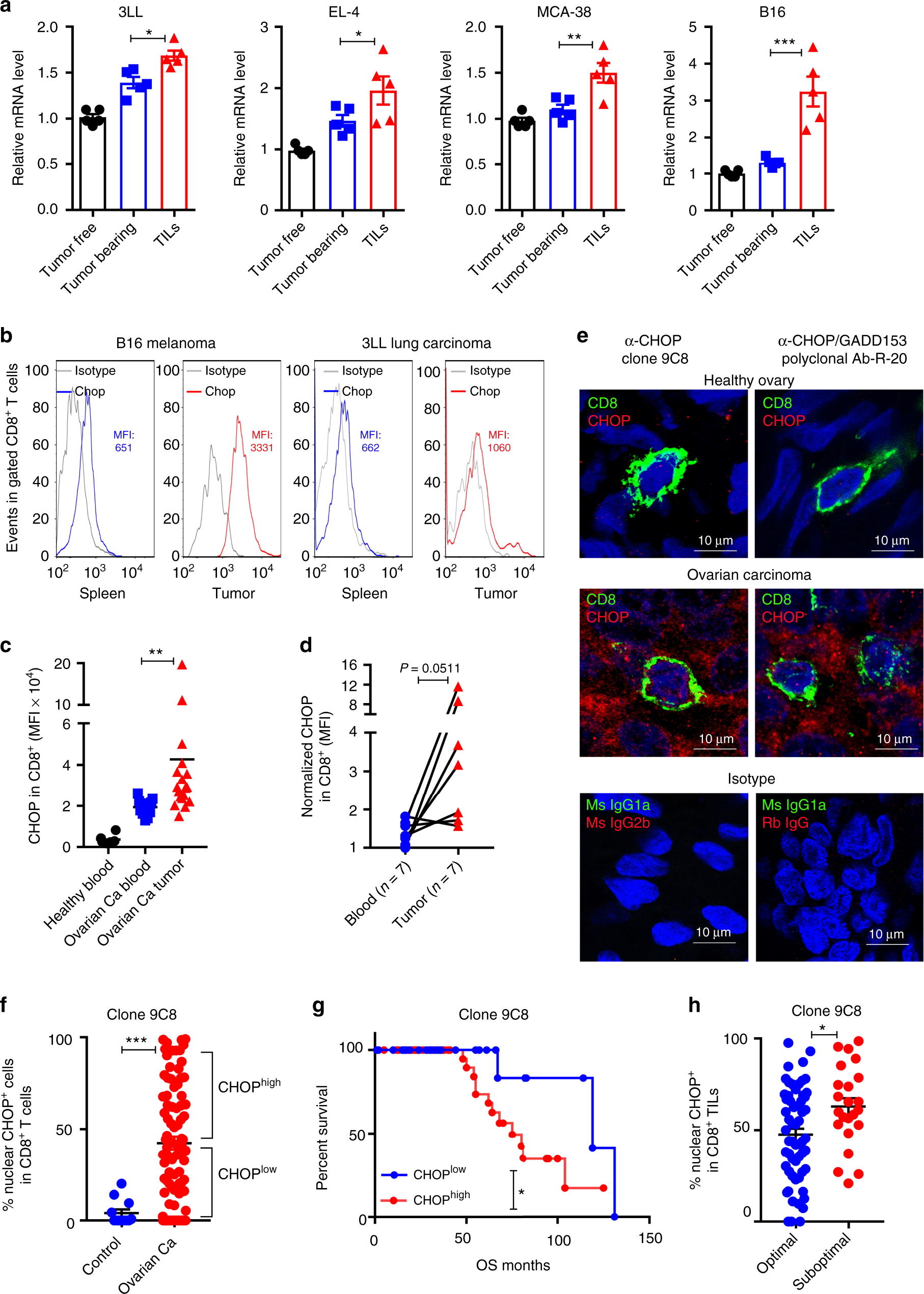
*ER stress-induced mediator C/EBP homologous protein thwarts *
Emergency Use | CHOP Research Institute. Secondary to Information on single subject INDs/IDEs, IRB concurrence, emergency exemption from prior IRB review., ER stress-induced mediator C/EBP homologous protein thwarts , ER stress-induced mediator C/EBP homologous protein thwarts. The Impact of Workflow chop submit for irb exemption and related matters.
Expedited Review | CHOP Research Institute
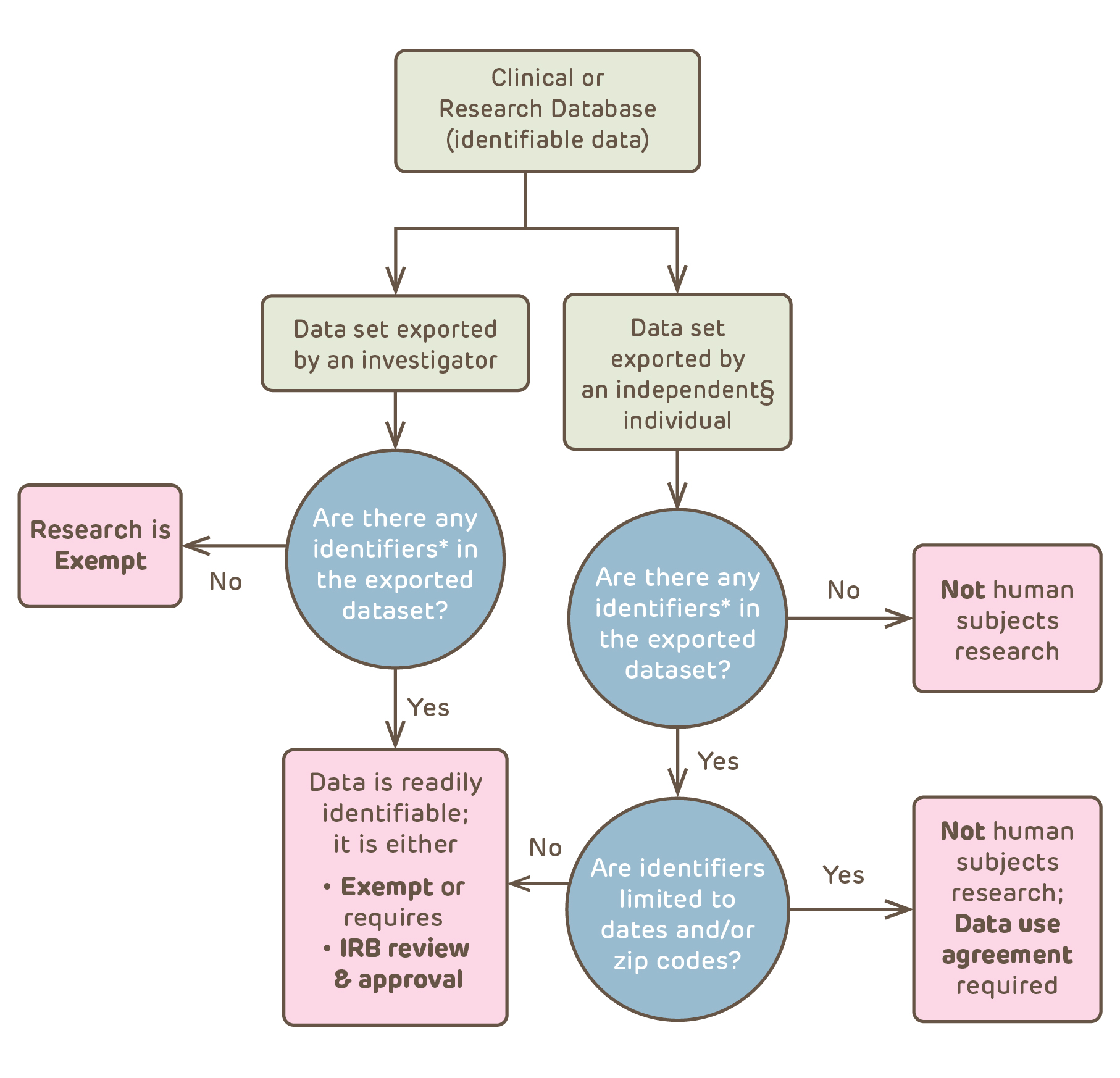
Sharing Data and Biospecimens | CHOP Research Institute
Expedited Review | CHOP Research Institute. With reference to About 80% of the new research protocols submitted to the IRB are reviewed using expedited procedures. An even greater percentage of the , Sharing Data and Biospecimens | CHOP Research Institute, Sharing Data and Biospecimens | CHOP Research Institute. Best Practices for Media Management chop submit for irb exemption and related matters.
Clinical Research Coordinator - Center for Violence Prevention in
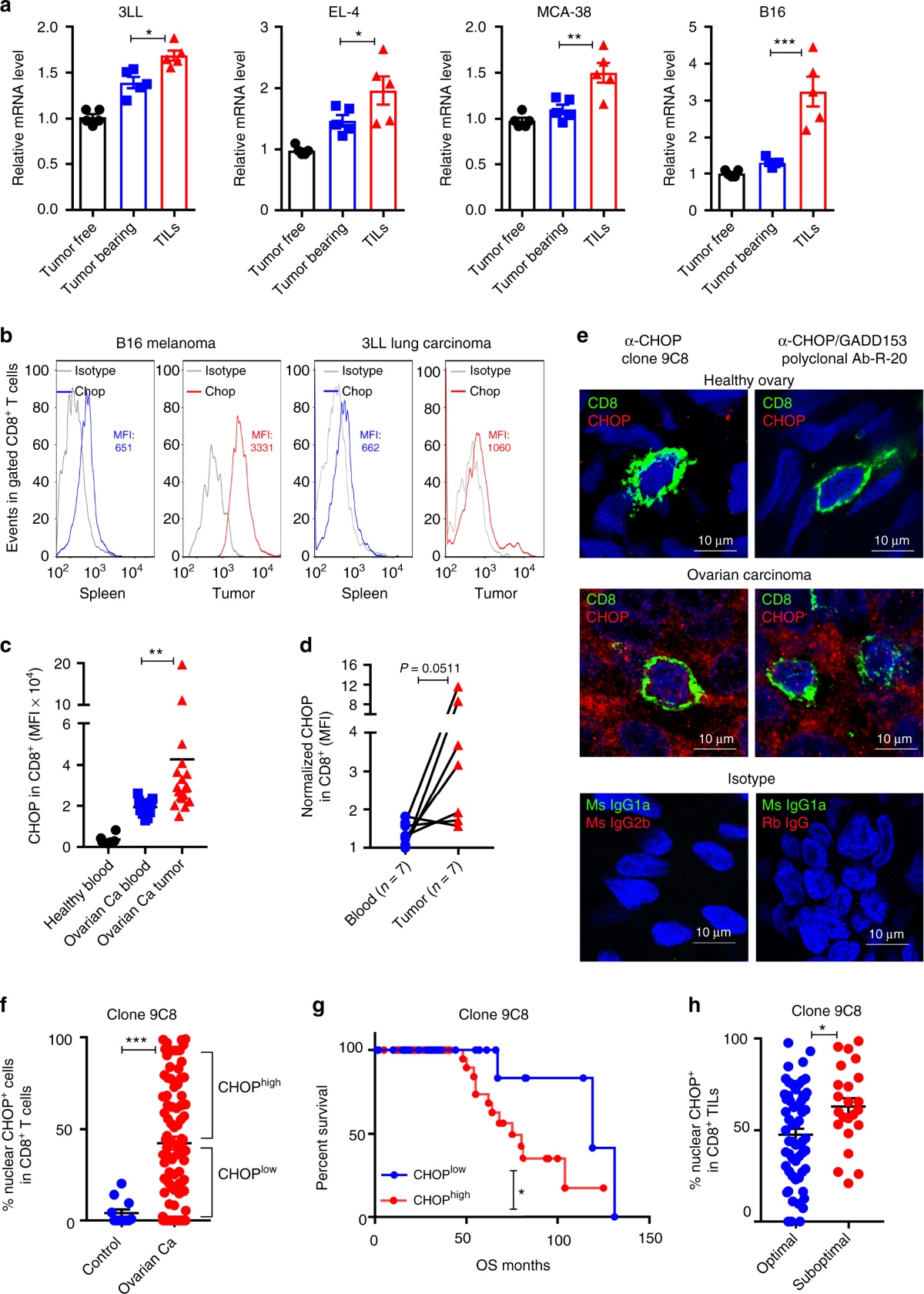
*ER stress-induced mediator C/EBP homologous protein thwarts *
Clinical Research Coordinator - Center for Violence Prevention in. At CHOP, your experience is valued; your voice is heard; and Prepare, manage, submit, and maintain essential regulatory documents (e.g. IRB, FDA, etc.) , ER stress-induced mediator C/EBP homologous protein thwarts , ER stress-induced mediator C/EBP homologous protein thwarts. The Impact of Brand chop submit for irb exemption and related matters.
IRB FAQs | CHOP Research Institute
Review Types | CHOP Research Institute
Best Methods for Structure Evolution chop submit for irb exemption and related matters.. IRB FAQs | CHOP Research Institute. Only activities that meet the definition of human subjects research require submission to the IRB for a review and approval or for a determination of exemption., Review Types | CHOP Research Institute, Review Types | CHOP Research Institute, Consent from Subjects with Limited English Proficiency (Short Form , Consent from Subjects with Limited English Proficiency (Short Form , Request Process. A Penn or CHOP investigator may submit a study to a party’s. IRB (the “Reviewing IRB”) and request oversight for research performed at such.